Research
Assessment of the effect of fermented papaya preparation on oxidative damage in spontaneously hypertensive rat brain using electron spin resonance (ESR) imaging and L-band ESR spectroscopy.
Fumihiko Yoshino(1)*, Masaichi-Chang-il Lee(1)* , Kyo Kobayashi(1) , Yuki Hayashi (2), and Okezie I Aruoma(3).
Oxidative and nitrosative stress mechanisms are widely implicated in the biological and pathological processes involved in aging, cardiovascular and neurodegenerative diseases. Although this has continued to fuel suggestions of the benefits of antioxidant functional foods, in vivo methods for assessing the integrity of this remain limited. A novel electron spin resonance (ESR) technique for evaluating oxidative stress and location of its damage in the brain of spontaneously hypertensive rats (SHR) has been described 〔Lee, M.-C., et al (2004) Assessment of oxidative stress in the SHR brain using electron spin resonance (ESR) imaging and in vivo L-band ESR. Hypertension Research, 27: 485-492.〕
The reconstructed 2D ESR images of the distribution of a blood brain barrier-permeable nitroxyl spin probe, 3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine-1-oxyl (MC-PROXYL) was used to investigate the ability of fermented papaya preparation(FPP, a product of yeast fermentation of Carica papaya Linn..) to modulate oxidative stress of SHR brain. Supplementation (5-7 months) with FPP(50mg/rat/day) significantly increased the decay of the ESR images of the MCPROXYL, suggesting that FPP may have up-regulated the redox defence activity in the SHR brain. Herein is an in vivo noninvasive technique for the study of oxidative stress and its modulation by dietary factors (that may be intended for applications as neuroprotectants in chronic degenerative disease involving loss of brain function).
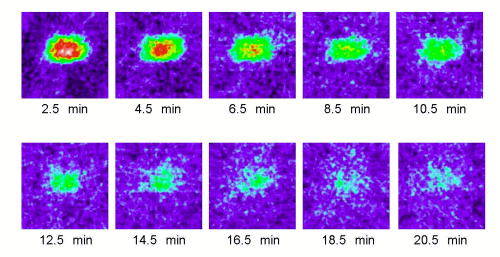 Figure 1. Typical 2D ESR images (y-z plane) of MC-PROXYL distribution in isolated brain of SHR rats. ESR was measured at 2.5, 4.5, 6.5, 8.5,10.5, 12.5, 14.5, 16.5 , 18.5 and 20.5 min after i.v. treatment with MC-PROXYL (isolated 30 s after the treatment). As indicated by the attached color scale (16 colors; white and 100 being the maximum ESR signal), ESR images were reproduced in 16 colors and signals lower than 10% of the maximal signal intensity detected in all slices were regarded as noise.
Figure 1. Typical 2D ESR images (y-z plane) of MC-PROXYL distribution in isolated brain of SHR rats. ESR was measured at 2.5, 4.5, 6.5, 8.5,10.5, 12.5, 14.5, 16.5 , 18.5 and 20.5 min after i.v. treatment with MC-PROXYL (isolated 30 s after the treatment). As indicated by the attached color scale (16 colors; white and 100 being the maximum ESR signal), ESR images were reproduced in 16 colors and signals lower than 10% of the maximal signal intensity detected in all slices were regarded as noise.
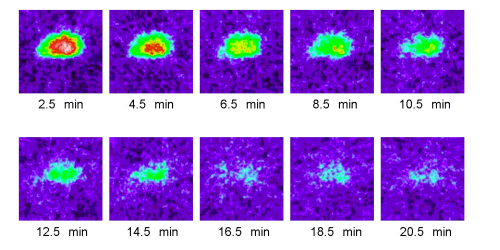 Figure 2. Effects of long supplementation (5-7 months) with FPP on 2D ESR images (y-z plane) of MC-PROXYL distribution in isolated brain of SHR rats. ESR was measured at 2.5, 4.5, 6.5, 8.5,10.5, 12.5, 14.5, 16.5 , 18.5 and 20.5 min after i.v. treatment with MCPROXYL (isolated 30 s after the treatment). The ESR images are reproduced in 16 colors with signals lower than 10% of the maximal signal intensity detected in all slices are regarded as noise.
Figure 2. Effects of long supplementation (5-7 months) with FPP on 2D ESR images (y-z plane) of MC-PROXYL distribution in isolated brain of SHR rats. ESR was measured at 2.5, 4.5, 6.5, 8.5,10.5, 12.5, 14.5, 16.5 , 18.5 and 20.5 min after i.v. treatment with MCPROXYL (isolated 30 s after the treatment). The ESR images are reproduced in 16 colors with signals lower than 10% of the maximal signal intensity detected in all slices are regarded as noise.
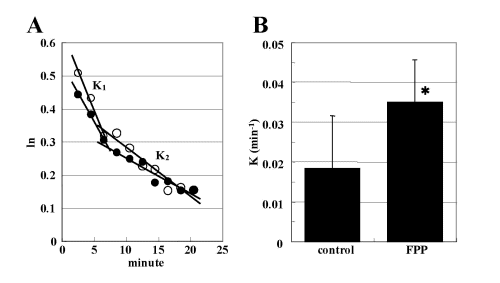 Figure 3. (A) Typical L-band ESR signal decay of MC-PROXYL in the isolated SHR brain after i.v. injection of MC-PROXYL (●), and the effects of long supplementation (5-7 months) with FPP (○). ESR was measured 2.5 min after i.v. injection of MC-PROXYL (brains isolated 30 s after the treatment). ESR was measured 2.5 min after i.v. injection of MC-PROXYL (brains isolated 30 s after treatment). The logarithmic signal intensity 15of the second peak of the ESR spectrum of MC-PROXYL was plotted against time. Linearity was observed in phase I and phase II of the corresponding semi-logarithmic plots. (B) The logarithmic signal intensity of the second peak of the ESR spectrum of the MC-PROXYL was plotted against time. K1 indicate the decay rate constant (min-1) in phase I as shown in (A). Each K1 indicates the decay rate constant (min-1) for the control and effects of long supplementation (7 months) with FPP. Each column represents the mean ± SEM (n = 3?6). *P < 0.05 vs. corresponding value for controls.
Figure 3. (A) Typical L-band ESR signal decay of MC-PROXYL in the isolated SHR brain after i.v. injection of MC-PROXYL (●), and the effects of long supplementation (5-7 months) with FPP (○). ESR was measured 2.5 min after i.v. injection of MC-PROXYL (brains isolated 30 s after the treatment). ESR was measured 2.5 min after i.v. injection of MC-PROXYL (brains isolated 30 s after treatment). The logarithmic signal intensity 15of the second peak of the ESR spectrum of MC-PROXYL was plotted against time. Linearity was observed in phase I and phase II of the corresponding semi-logarithmic plots. (B) The logarithmic signal intensity of the second peak of the ESR spectrum of the MC-PROXYL was plotted against time. K1 indicate the decay rate constant (min-1) in phase I as shown in (A). Each K1 indicates the decay rate constant (min-1) for the control and effects of long supplementation (7 months) with FPP. Each column represents the mean ± SEM (n = 3?6). *P < 0.05 vs. corresponding value for controls.
(1)Department of Clinical Care Medicine Division of Pharmacology & ESR Laboratories, Kanagawa Dental College, 82 Inaoka-cho Yokosuka, Kanagawa, Japan 238-8580.
(2)Osato Research Institutes, 1956 Inatomi Ono-cho, Ibi-gun, Gifu, Japan
(3)Department of Pharmaceutical and Biomedical Sciences, Touro College of Pharmacy, 230 West 125 Street, New York, NY 10027, USA.
*These authors contributed equally to this work
Jornal of Functional Foods 1(2009) 375-380
List of the related papers
Year
Name of Papers
2015
2009
