Research
THE HIDDEN PHENOMENON OF OXIDATIVE STRESS DURING TREATMENT OF SUBCLINICAL-MILD HYPOTHYROIDISM: A PROTECTIVE NUTRACEUTICAL INTERVENTION
FILE2014
Category Thyroid glandHealthy individualBasic researchClinical research
THE HIDDEN PHENOMENON OF OXIDATIVE STRESS DURING TREATMENT OF SUBCLINICAL-MILD HYPOTHYROIDISM: A PROTECTIVE NUTRACEUTICAL INTERVENTION
Rejuvenation Res. 17(2):180-183,2014
It is known that thyroid hormones are associated with the redox-balance homeostatic regulation. Indeed, thyroid dysfunctions increase lipoperoxides which is an autocatalytic mechanism leading to oxidative damage of cellular membranes. Increased ROS is also a feature of hyperthyroidism, this might be one of the reasons behind the discomfort and loss of working activity often experienced in these patients. Thus, the aim of the present study was to test a redox balance modulator, FPP, in association with treatment of SH or mild hypothyroidism (MH) in terms of: clinical symptom score, oxidative stress, lipid profile and gene expression involved in thyroid regulation.
60 generally healthy females, aged 18-55, presented with SH or MH were put on a 2-week wash-out period and then divided into two groups (30 each) matched as for age, routine biochemical status, dietary profile and thyroid status assessment. Both groups received similar medical treatment for their hypothyroidism. One group was given FPP 3g 1 sachet twice-a-day for 3 months (FPP group), while the other group was given a flavored sugar sachet as placebo (placebo group). A matched group of normal thyroid function subjects was our healthy control (HC).
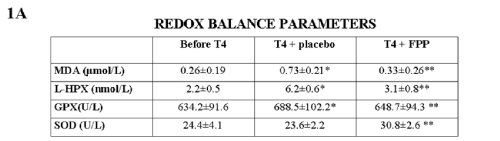
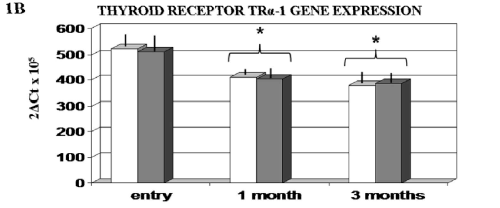
Lipid profile and thyroid hormone parameters remained unchanged and unaffected by FPP supplementation. As compared to HC a significant increase of all oxidative markers was observed in MH subjects (p<0.05) but not in SH. Treatment with T4 brought about a further increase (p<0.05 vs baseline) in MH and a significant increase also in SH subjects. While placebo was ineffective, FPP-supplemented individuals showed a significant normalization of redox markers in all tested subjects (Fig.1). All 3 subjects in the FPP-treated group reporting a long-standing gastrointestinal discomfort often requiring to lower the dosage of the T3 therapy when cyclically being prescribed this in association to their established T4, reported a complete recovery. THS cased a significant down-regulation of TRα-1 mRNA (p<0.01 vs baseline) (Fig.2) but not of TRβ-1 genes and this pattern was unaffected by FPP.
It appears that not only FPP can counteract the thyroid hormone-induced oxidative stress but it does not impair the physiological primary hormone-related receptors. Thus, FPP intervention might be an advisable integrative treatment to be associated to long-standing THS regimens.
List of the related papers
Year
Name of Papers
2014
